Our mission is to develop, manufacture, and market a targeted, proprietary means of preventing anti-drug antibodies in NHP during the testing of biotherapeutics. Our approach will:
- Improve the robustness and quality of preclinical PK and Toxicology testing
- Reduce the cost of NHP testing
- Advance the 3Rs
Our preclinical platform is founded on the clinical research findings of our scientific founder, an internationally recognized expert in the field of immunology and ADA, and his recognition of the importance of extending those results to the preclinical testing of biotherapeutics in NHP in order to both improve testing and promote animal welfare.
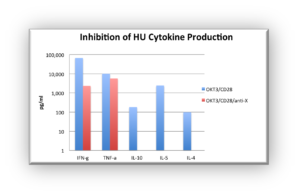
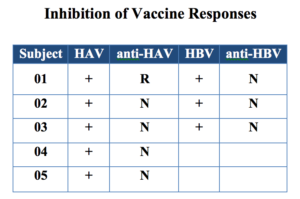
In preclinical and clinical studies respectively, it was demonstrated that anti-X abolished the production of cytokines important for antibody production and blocked the response to the Hepatitis A and B vaccines without increasing the risk of infection or other adverse events.
Our business team has a proven record of successful business development, having founded and played key management roles in a number of international startups including:
Space Partnership International (“SPI”), LLC, a hands-on management-consulting group with deep, end-to-end expertise across business, regulatory, engineering and risk management disciplines, helping governments and companies to develop, implement and grow their own space-based scientific research, telecommunications, Earth observation and Weather systems (www.spacepi.com);
Assure Space, which is now a major provider of space insurance capacity for launch and in-orbit risks (www.assure-space.com), and recently sold to AmTrust, a multi-billion dollar insurance company;
Driversiti, a proprietary mobile-based platform that uses the sensors in smartphones and tablets to deliver Situational Awareness to drivers (www.driversiti.com);
AfriHUB, which over the past 10 years has become the largest human capacity building business in West Africa, having trained more than more than 1.5 million students in IT career paths based on internationally accepted testing (www.AfriHub.com).
Our Team
JoMoCo was founded by a team of experts in their respective fields:
Jean-Michel Eid
Jean-Michel Eid, Co-founder: Mr. Eid is a seasoned executive with over 25 years of experience in establishing, managing and growing technology-related companies in the US, Europe, Asia and Africa. At JoMoCo, he is responsible for overseeing all financial, capital market and business development activities.
Mr. Eid started JoMoCo with a former Chief Medical Research Officer in the Laboratory of Immunology of the Office of Biotechnology Products at the Food and Drug Administration (FDA), who while at the FDA observed the adverse impact that anti-drug antibodies (ADA) have on the outcomes of both preclinical and clinical studies. Capitalizing on observations he had previously made as a Senior Clinical Investigator at the National Institutes of Health (NIH), he developed a selective method that is predicted to effectively prevent the development of ADA in nonhuman primates. He holds M.D. and Ph.D. degrees from the Johns Hopkins University and School of Medicine. His work and publications span the fields of gene regulation, retrovirology, gene therapy, immune activation, the immunogenicity of biotherapeutics, and clinical trials in immune tolerance. A diplomate of the American Board of Allergy and Immunology, he continues to serve at the NIH as a Special Volunteer, conducting research to improve the safety of CAR-T cell therapies.
As a founding member of Space Partnership International, LLC, Mr. Eid continues to provide business development, financial, risk management services for the investment, insurance communities and commercial start-ups.
Mr. Eid began his career in the financial division of Arianespace Inc. Subsequent to Ariane, Mr. Eid was engaged in finance, space insurance and risk management activities first with International Space Brokers (Willis), and later as a Managing Director for Aon. In addition he founded and played key management roles in a number of international start-ups, including Assure Space, which is now a major provider of space insurance capacity for launch and in-orbit risks (www.assure-space.com), and recently sold to AmTrust, a multi-billion dollar insurance company; Driversiti, a proprietary mobile-based platform that uses the sensors in smartphones and tablets to deliver Situational Awareness to drivers (www.driversiti.com);
Mr. Eid holds an M.B.A. degree in International Finance from George Washington University and a B.S. in Business Administration from Georgetown University.
Dr. Steve Swanson
Dr. Swanson has more than 25 years of pharmaceutical and biotechnology experience, with a specialty in immunology, and has been an industry leader in advancing global standards for immunogenicity assessment.
Prior to joining JoMoCo, Dr. Swanson spent 15 years at Amgen as Department Head for Clinical Immunology, a then-new department providing immunogenicity and cytometry support for all of Amgen's therapeutic proteins. Prior to joining Amgen, he led the immunoassay laboratory in the Biotechnology department at Schering Plough Research Institute.
Dr. Swanson has been actively involved in multiple industry professional associations, including the American Association of Pharmaceutical Scientists (AAPS), where he is a Fellow, and was a co-author of AAPS-sponsored Industry White Papers that were incorporated into FDA and EMA Guidance for Immunogenicity Assessment. He was also an industry representative for the EMA Committee that developed the first Immunogenicity Recommendations and was engaged by the FDA to train reviewers on immunogenicity assessment. Dr. Swanson has authored more than 60 publications.
He holds a BA in chemistry/biology from North Central College, a PhD in microbiology from the University of Iowa, and completed a post-doctoral fellowship at The Ohio State University.
Bonnie Rup
Dr. Rup has over 25 years of leadership experience in development of biopharmaceuticals with a specialty in immunogenicity risk assessment and regulatory aspects of biopharmaceutical development.
Prior to joining JoMoCo’s Scientific Advisory Board, Ms. Rup spent 25 years at Pfizer as a Research Fellow in the Immunogenicity Sciences Discipline, PDM-New Biological Entities. She led the Immunogenicity Sciences group within PDM responsible for overseeing implementation of PDM and the Global Pfizer immunogenicity strategy supporting Pfizer's Biotherapeutic Pipeline (co-lead for Pfizer Immunogenicity Expert Working Group). Prior to her time at Pfizer, she was the Asst. Vice President for Protein Bioanalytics and Drug Safety at Wyeth Research.
She received her Ph.D. in Microbiology and Immunology from the Univ. of Texas at Austin and her B.S. in Microbiology from the Univ. of Massachusetts Amherst.
